REACH试验再分析:哪些肝细胞癌患者可从RAM治疗获益?
Ramucirumab(Cyramza,礼来)是特异性阻断血管内皮生长因子受体2(VEGFR2)及下游血管生成相关通路的人源化单克隆抗体。美国食品药物管理局(FDA)于2014年4月批准ramucirumab用于治疗晚期胃癌或胃食管结合部腺癌。
Ramucirumab在肝癌领域还会再显身手吗?一项全球性的、多中心、3期研究评估了RAM单药治疗既往接受过索拉非尼治疗的晚期肝细胞癌患者的疗效和安全性。REACH试验的主要结果在2014年ESMO大会上展出。对该研究中特定亚组的分析结果即将在1月15日-17日在美国旧金山召开的2015年ASCO胃肠道肿瘤(GI)研讨会上公布,小编提前和大家分享这项精彩研究。
研究背景
REACH试验是一项全球性的、多中心、随机、双盲、3期研究,旨在评估RAM单药治疗既往接受过索拉非尼治疗的晚期肝细胞癌患者的疗效和安全性。REACH试验的主要结果在2014年ESMO大会上展出。ITT人群(RAM组283例,安慰剂组[PBO]282例)总生存期(OS)的HR为0.866(95%CI 0.717,1.046,P=0.1391); RAM组中位OS为9.2个月,安慰剂组中位OS为7.6个月。对预先指定亚组的基线AFP(临界值400 ng/mL)分析表明,AFP是RAM带来生存获益的预测指标。
研究方法
在基线AFP以400 ng/mL为临界值的基础上开展预先指定的亚组分析。采用分层/不分层的Cox回归分析模型和相应的时序检验进行附加的分析,以评估基线AFP和RAM治疗效果的相关性。
研究结果
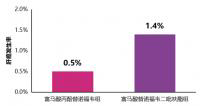
在250例基线AFP≥400 ng/mL的患者中(RAM组119; PBO组131),总生存期的HR为0.67(95%CI 0.51-0.90; P =0.0059)。RAM组中位OS是7.8个月,PBO组中位OS是4.2个月。在417例基线AFP≥1.5×正常上限的患者中(ULN; RAM组205; PBO组212),RAM组中位OS是8.6个月,PBO组中位OS是5.7个月,HR为0.749(95%CI:0.603,0.930)(P =0.0088)。对基线AFP和RAM疗效的关联性的检测(使用两个临界值[400 ng / mL和1.5 xULN])是有意义的(p分别=0.0272和0.0372)。RAM在这些人群的安全性与在总的安全人群中观察到的安全性相似。附加的REACH分析表明,基线AFP对RAM带来OS获益的预测价值将被公布。
结论
在基线AFP≥400 ng/mL或≥1.5×ULN的患者中观察到了具有临床意义的OS改善。附加的分析表明,基线AFP可能是RAM治疗带来OS获益的一个预测指标。
英文摘要
Ramucirumab (RAM) as second-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): Analysis of patients with elevated α-fetoprotein (AFP) from the randomized phase III REACH study.(Abstract No:232)Background: REACH was a global, multicenter, randomized, double-blind, phase 3 study evaluating the efficacy and safety of RAM as a single agent for the treatment of pts with advanced HCC after prior sorafenib therapy. The primary outcome for REACH was presented at ESMO 2014. The overall survival (OS) HR for the ITT population (RAM 283; placebo [PBO] 282) was 0.866 (95% CI 0.717, 1.046; p=0.1391); median OS was 9.2m for RAM vs 7.6m for PBO. The pre-specified subgroup **ysis of baseline AFP (cutoff 400 ng/mL) suggested AFP is a predictive marker for RAM survival benefit.
Methods: Pre-specified subgroup **ysis was performed based on baseline AFP with a cutoff of 400 ng/mL. Additional **yses were conducted using stratified/unstratified cox regression models and corresponding log rank test to evaluate the relationship between baseline AFP and RAM treatment effect.
Results: In 250 pts with baseline AFP ≥400 ng/mL (RAM 119; PBO 131), OS HR was 0.67 (95% CI 0.51–0.90; p=0.0059)。 Median OS was 7.8m for RAM vs 4.2m for PBO. In 417 pts with a baseline AFP ≥1.5 × upper limit of normal (ULN; RAM 205; PBO 212), mOS was 8.6m for RAM vs 5.7m for PBO and the HR was 0.749 (95% CI: 0.603, 0.930) (p=0.0088)。 The interaction testing of baseline AFP and RAM treatment effect on OS using both cutoffs (400 ng/mL and 1.5 xULN) are significant (p-value = 0.0272 and 0.0372, respectively)。 The safety profile in these pt populations was similar to that observed in the overall safety population. Additional REACH **yses demonstrating the predictive value of baseline AFP for RAM treatment effect on OS will be presented.
Conclusions: A clinically meaningful improvement in OS was observed in populations with a baseline AFP ≥400 ng/mL or ≥1.5 × ULN. Additional **yses demonstrated a consistent RAM OS benefit for the pt population with baseline AFP over a wide range of values above the normal range. Baseline AFP is a likely predictive marker for RAM OS benefit.
本站所注明来源为"爱爱医"的文章,版权归作者与本站共同所有,非经授权不得转载。
本站所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,不希望被转载的媒体或个人可与我们
联系zlzs@120.net,我们将立即进行删除处理
热点图文
-
AASLD2018研究进展丨慢乙肝患者治疗期间的肾脏安全管理
在新药取得成功之前,应用口服核苷(酸)类似物(NA)治疗慢性乙型肝炎(简称...[详细]
-
快讯丨TAF治疗4年的肝细胞癌发生率低于TDF
5月17日,在第十届全国疑难及重症肝病大会上,我国香港大学司徒伟基教授交流...[详细]